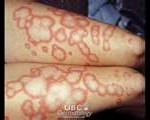
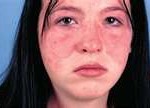
Lupus is a critically serious disease that makes the immune system attack the body’s own tissue and organs.
It most often affects women and usually develops at their teens and forties. It has a range of symptoms that includes arthritis, kidney damage, chest pain, hair loss, fatigue and a butterfly-shaped rash across the cheeks and nose. The organ damage can be critical.
The new drug introduced, Benlysta from Human Genome Sciences Inc,is the first new treatment in fifty years for the autoimmune disease lupus.
It has won US approval for certain lupus patients already receiving standard therapy. It is given once a month by intravenous infusion.
In a company-funded study, 43 percent of patients given a high Benlysta dose with standard therapies felt relief and no disease worsening in various organs noted after one year of treatment.
Compared to that of nearly 34 percent with a placebo and standard care of treatment.
Doctors hail Benlysta as an advance but note it will not work for everyone and more treatments are needed. Some experts have described Benlysta’s effects as mild.
The Food and Drug Administration noted Benlysta did not appear to help black patients in clinical studies but there were not enough of them to draw a firm conclusion.
The agency is requiring a post-approval study to further assess results in black patients.
Black women have a three times higher incidence of lupus than white women, the FDA said.
The most common side effects in Benlysta studies included nausea, diarrhea and fever.
More deaths and serious infections were reported with Benlysta compared with a placebo. Studies showed 0.9 percent of patients died after taking the highest Benlysta dose, compared with 0.4 percent in the placebo group.
Some other option for lupus treatment are older drugs that work for some patients but can carry harsh side effects such as severe bone loss from steroids.
Many are not approved for lupus. Non-steroidal anti-inflammatory drugs, steroids and immuno-suppressants often are prescribed.
But Human Genome is developing a new version of Benlysta that could be given by injection rather than intravenously.
Another drug, epratuzumab from UCB and Immunomedics Inc, is in late-stage studies. Earlier research is ongoing for other potential therapies.